What Is the Noble Gas Configuration for Mercury
Xenon are all noble gas. The electron configuration of mercury is.

Webelements Periodic Table Mercury Properties Of Free Atoms
So for sodium we make the substitution of left ce Ne right for the 1s2 2s2 2p6 part of the configuration.
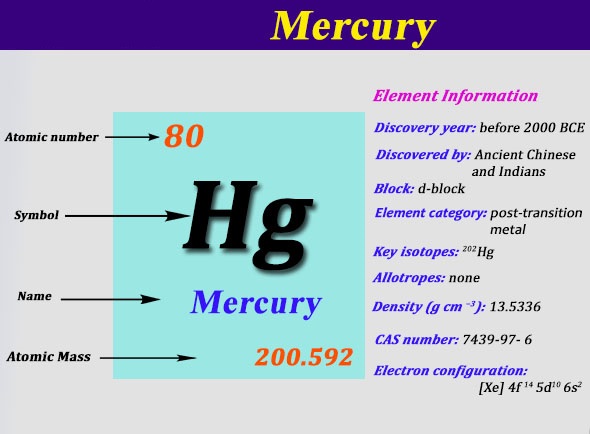
. Noble gas configuration for mercury Vocabulary inert pair lattice metal oxidize valence electron Mercury is puzzling in several ways. The noble gas notation for silicon atom is. So the noble gas notation for.
If Element X Is A Noble Gas What Will Be The Symbol For The. Back to key information about the element. Sodiums noble gas configuration becomes left ceNe right 3s1.
Mercury is much less reactive than cadmium or zinc. Mercury-200 is composed of 80 protons 120 neutrons and 80 electrons. Sodiums noble gas configuration becomes Ne3 s1.
What is the Noble Gas Configuration for Lead. Xe 6s2 4f14 5d10 6p2. Its a liquid at room temperature and pressure but all of its neighbors on the periodic table are solids.
Noble gas notation is defined as the notation in which the configuration of an atom is written in terms of previous noble gas. Mercury Mercury is a silvery metal that is liquid at standard temperature and pressure STP. Of the elemental symbol of the last noble gas prior to that atom followed by the configuration of the remaining electrons.
For the 6th row of the periodic table we introduce the 4f orbitals and proceed to atoms having occupied 5d orbitals. Mercury-201 is composed of 80 protons 121 neutrons and 80 electrons. Mercury is having an atomic number of 80.
Top best answers to the question Noble gas configuration for tin Answered by Elouise Monahan on Fri Jul 9 2021 1143 PM The noble gas Krypton has an electron configuration of 1s22s22P63s23p64s23d104p6 So we can replace this portion of tins electron configuration with the noble gas notation Kr This makes the electron configuration for Tin -. The mercuric ion Hg and the mercurous ion that would be expected to be Hg but is Hg-Hg. Mercury-198 is composed of 80 protons 118 neutrons and 80 electrons.
So for sodium we make the substitution of left ceNe right for the 1s2 2s2 2p6 part of the configuration. What is the Noble Gas Configuration for Potassium. What Is the Noble Gas Configuration for Mercury Get link.
The weak bonds formed by these elements become solids which melt easily at relatively low temperatures. You find the electron configuration which is 1s2 2s2 2p2 3s2 3p3 4s2 3d6. 1s2 2s2 2p6 3s2 3p6 4s2 3d10 4p6 5s2 4d10 5p6 6s2 4f14 5d10.
Oganesson Og is a synthetically produced highly radioactive element variously predicted to be another noble gas or to break the trend and be reactive due to relativistic effects. As indicated in this formula mercury has 80 electrons with two electrons on its outer energy level. Oct 29 2017.
A noble gas configuration of an atom consists of the elemental symbol of the last noble gas prior to that atom followed by the configuration of the remaining electrons. The chemical symbol for Mercury is Hg. Anonymous answered Xe 6s2 4f14 5d10.
Other Apps - April 13 2022. The condensed or the shortened form of the electronic configuration is. Solved Write The Noble Gas Notation For The Electron Configu Rations Of Each Of The Following Elements A As E Sn B Pb F Xe C Lr G La D Hg.
So for sodium we make the substitution of Ne for the 1 s2 2 s2 2 p6 part of the configuration. An electron configuration is the arrangement of electrons in the various. Possible oxidation states are 12.
It has a Xe core so in shorthand notation you can include Xe instead of 1s22s22p63s23p63d104s24p64d105s25p6 for 54 electrons. 2 8 18 32 18 2. You should then find its atomic number is 80.
What is the Noble Gas Configuration for Neon. Mercury-196 is composed of 80 protons 116 neutrons and 80 electrons. Mercury-199 is composed of 80 protons 119 neutrons and 80 electrons.
Electron configuration chart of all Elements is mentioned in the table belowThe Shorthand electron configuration or Noble gas configuration as well as Full. The full electron configuration of mercury is 1s2 2s2p6 3s2p6d10 4s2p6d10f14 5s2p6d10 6s2. This is the formula of a mercury atom in a neutral state without the charge of more or less electrons.
1s 2 2s 2 2p 6 3s 2 3p 6 3d 10 4s 2 4p 6 4d 10 5s 2 5p 6 4f 14 5d 10 6s 2. Examine your periodic table and find mercury. Whats The Noble Gas Notation For Mercury.
Mercury has a unique electronic configuration which strongly resists removal of an electron making mercury behave similarly to noble gas elements. A noble gas configuration of an atom consists of the elemental symbol of the last noble gas prior to that atom followed by the configuration of the remaining electrons. So for sodium we make the substitution of Ne for the 1s22s22p6 part of the configuration.
Three Elements X Y And Z Have Consecutive Increasing Atomic Numbers. The six naturally occurring noble gases are helium He neon Ne argon Ar krypton Kr xenon Xe and the radioactive radon Rn. Electron configuration of Mercury Hg Xe 4f 14 5d 10 6s 2.
A noble gas configuration of an atom consists of the elemental symbol of the last noble gas prior to that atom followed by the configuration of the remaining electrons. Sodiums noble gas configuration becomes. The electron configuration of Hg.
Xe 4f14 5d10 6s2. Electron configuration of Mercury is Xe 4f14 5d10 6s2. Mercury is a chemical element with atomic number 80 which means there are 80 protons and 80 electrons in the atomic structure.
1s 2 2s 2 2p 6 3s 2 3p 6 3d 10 4s 2 4p 6 4d 10 5s 2 5p 6 4f 14 5d 10 6s 2. Mercury forms two ions. Silicon is the 14th element of the periodic table having 14 electrons.
1s2 2s2 2p6 3s2 3p6 3d10 4s2 4p6 4d10 5s2 5p6 4f14 5d10 6s2. Sodiums noble gas configuration becomes Ne3s1. What is the Electron Configuration for Mercury.
Electron Configuration and Oxidation States of Mercury. The nearest noble gas to this element is neon.

How To Write The Electron Configuration For Hg And Hg 2 Youtube

Comments
Post a Comment